Can H2s Form Hydrogen Bonds
Hydrogen sulfide forms hydrogen bonds, scientists have found. Their discovery disproves 1954 Nobel laureate Linus Pauling's belief that, in its solid state, HtwoS is fundamentally different from HiiO.
In his influential textbook, The Nature of the Chemical Bond, Pauling wrote that ice is structured by hydrogen bonds whereas solid hydrogen sulfide'due south structure is controlled by van der Waals interactions. The latter are purely electrostatic interactions between dipoles whereas the former involve shared electron density similar to covalent bonds.
Pauling based his conclusions on the fact that solid HiiS and water ice await very different in their bulk state. In H2S, each molecule is surrounded by 12 others, whereas in ice, each molecule has only four neighbours.
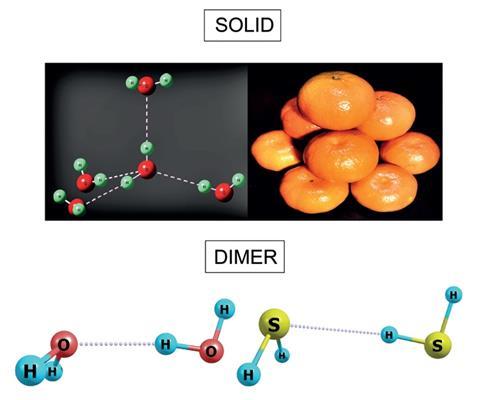
But at ultra-depression temperatures, hydrogen sulfide forms dimers just like water does. And merely like h2o, the chemical compound responsible for rotten eggs' olfactory property forms hydrogen bonds, Elangannan Arunan from the Indian Found of Science and colleagues have found.
Their microwave spectra, recorded at 3K (–270°C), are the start evidence of hydrogen bonding in H2S. They showed that the hydrogen on one molecule is closer to its neighbour'due south sulfur atom than their combined atomic radii would suggest. Moreover, the two atoms are arranged in an most linear fashion – both tell-tale signs of hydrogen bonding. Arunan's team confirmed their findings with theoretical calculations.
Can H2s Form Hydrogen Bonds,
Source: https://www.chemistryworld.com/news/hydrogen-sulfide-surprises-as-its-discovered-to-have-hydrogen-bonds/3009753.article
Posted by: mcguiganselse2000.blogspot.com


0 Response to "Can H2s Form Hydrogen Bonds"
Post a Comment