Thallium Has Two Stable Isotopes
| |||||||||||||||||||||||||||||||
| Standard diminutive weight A r°(Tl) |
| ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
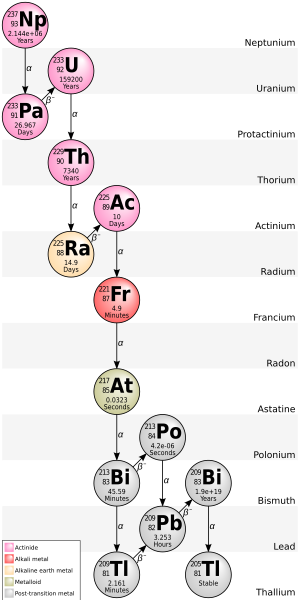
Thallium (81Tl) has 41 isotopes with atomic masses that range from 176 to 216. 203Tl and 205Tl are the only stable isotopes and 204Tl is the most stable radioisotope with a one-half-life of 3.78 years. 207Tl, with a one-half-life of iv.77 minutes, has the longest half-life of naturally occurring Tl radioisotopes. All isotopes of thallium are either radioactive or observationally stable, meaning that they are predicted to be radioactive simply no actual decay has been observed.
Thallium-202 (half-life 12.23 days) can exist made in a cyclotron[three] while thallium-204 (half-life iii.78 years) is made past the neutron activation of stable thallium in a nuclear reactor.[four]
In the fully ionized state, the isotope 205Tl becomes beta-radioactive, decomposable to 205Lead,[5] but 203Tl remains stable.
205Tl is the decay product of bismuth-209, an isotope that was once thought to be stable merely is now known to undergo alpha decay with an extremely long half life of 2.01×1019 y.[6] 205Tl is at the terminate of the neptunium series decay concatenation.
List of isotopes [edit]
| Nuclide[7] [n ane] | Historic name | Z | Due north | Isotopic mass (Da) [eight] [northward 2] [n three] | Half-life [n 4] | Decay fashion [north v] | Daughter isotope [n 6] | Spin and parity [n 7] [n 4] | Natural abundance (mole fraction) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Excitation energy[n 4] | Normal proportion | Range of variation | |||||||||||||||||
| 176Tl | 81 | 95 | 176.00059(21)# | 5.2(+30−14) ms | (3−, 4−, 5−) | ||||||||||||||
| 177Tl | 81 | 96 | 176.996427(27) | 18(5) ms | p | 176Hg | (i/2+) | ||||||||||||
| α (rare) | 173Au | ||||||||||||||||||
| 177mTl | 807(18) keV | 230(40) μs | p | 176Hg | (11/ii−) | ||||||||||||||
| α | 173Au | ||||||||||||||||||
| 178Tl | 81 | 97 | 177.99490(12)# | 255(10) ms | α | 174Au | |||||||||||||
| p (rare) | 177Hg | ||||||||||||||||||
| 179Tl | 81 | 98 | 178.99109(5) | 270(30) ms | α | 175Au | (1/2+) | ||||||||||||
| p (rare) | 178Hg | ||||||||||||||||||
| 179mTl | 860(30)# keV | 1.threescore(xvi) ms | α | 175Au | (9/2−) | ||||||||||||||
| IT (rare) | 179Tl | ||||||||||||||||||
| 180Tl | 81 | 99 | 179.98991(xiii)# | i.v(2) south | α (75%) | 176Au | |||||||||||||
| β+ (25%) | 180Hg | ||||||||||||||||||
| EC, fission (10−4%) | 100Ru, 80Kr [ix] | ||||||||||||||||||
| 181Tl | 81 | 100 | 180.986257(ten) | three.two(3) southward | α | 177Au | 1/2+# | ||||||||||||
| β+ | 181Hg | ||||||||||||||||||
| 181mTl | 857(29) keV | one.vii(four) ms | α | 177Au | ix/2−# | ||||||||||||||
| β+ | 181Hg | ||||||||||||||||||
| 182Tl | 81 | 101 | 181.98567(8) | two.0(3) s | β+ (96%) | 182Hg | 2−# | ||||||||||||
| α (4%) | 178Au | ||||||||||||||||||
| 182m1Tl | 100(100)# keV | ii.9(5) s | α | 178Au | (seven+) | ||||||||||||||
| β+ (rare) | 182Hg | ||||||||||||||||||
| 182m2Tl | 600(140)# keV | 10− | |||||||||||||||||
| 183Tl | 81 | 102 | 182.982193(10) | half dozen.ix(seven) s | β+ (98%) | 183Hg | i/ii+# | ||||||||||||
| α (two%) | 179Au | ||||||||||||||||||
| 183m1Tl | 630(17) keV | 53.3(3) ms | IT (99.99%) | 183Tl | 9/2−# | ||||||||||||||
| α (.01%) | 179Au | ||||||||||||||||||
| 183m2Tl | 976.viii(three) keV | 1.48(10) μs | (13/2+) | ||||||||||||||||
| 184Tl | 81 | 103 | 183.98187(v) | ix.7(6) s | β+ | 184Hg | 2−# | ||||||||||||
| 184m1Tl | 100(100)# keV | ten# s | β+ (97.ix%) | 184Hg | 7+# | ||||||||||||||
| α (2.1%) | 180Au | ||||||||||||||||||
| 184m2Tl | 500(140)# keV | 47.1 ms | Information technology (99.911%) | (10−) | |||||||||||||||
| α (.089%) | 180Au | ||||||||||||||||||
| 185Tl | 81 | 104 | 184.97879(6) | 19.5(5) s | α | 181Au | ane/ii+# | ||||||||||||
| β+ | 185Hg | ||||||||||||||||||
| 185mTl | 452.8(twenty) keV | 1.93(8) s | Information technology (99.99%) | 185Tl | ix/2−# | ||||||||||||||
| α (.01%) | 181Au | ||||||||||||||||||
| β+ | 185Hg | ||||||||||||||||||
| 186Tl | 81 | 105 | 185.97833(20) | 40# s | β+ | 186Hg | (2−) | ||||||||||||
| α (.006%) | 182Au | ||||||||||||||||||
| 186m1Tl | 320(180) keV | 27.v(ten) s | β+ | 186Hg | (7+) | ||||||||||||||
| 186m2Tl | 690(180) keV | ii.9(2) due south | (ten−) | ||||||||||||||||
| 187Tl | 81 | 106 | 186.975906(ix) | ~51 s | β+ | 187Hg | (one/two+) | ||||||||||||
| α (rare) | 183Au | ||||||||||||||||||
| 187mTl | 335(3) keV | 15.60(12) s | α | 183Au | (nine/2−) | ||||||||||||||
| IT | 187Tl | ||||||||||||||||||
| β+ | 187Hg | ||||||||||||||||||
| 188Tl | 81 | 107 | 187.97601(4) | 71(two) due south | β+ | 188Hg | (2−) | ||||||||||||
| 188m1Tl | 40(30) keV | 71(1) south | β+ | 188Hg | (seven+) | ||||||||||||||
| 188m2Tl | 310(30) keV | 41(4) ms | (nine−) | ||||||||||||||||
| 189Tl | 81 | 108 | 188.973588(12) | 2.3(two) min | β+ | 189Hg | (1/ii+) | ||||||||||||
| 189mTl | 257.6(13) keV | 1.four(1) min | β+ (96%) | 189Hg | (9/2−) | ||||||||||||||
| IT (4%) | 189Tl | ||||||||||||||||||
| 190Tl | 81 | 109 | 189.97388(5) | two.6(three) min | β+ | 190Hg | 2(−) | ||||||||||||
| 190m1Tl | 130(xc)# keV | 3.7(three) min | β+ | 190Hg | 7(+#) | ||||||||||||||
| 190m2Tl | 290(70)# keV | 750(40) μs | (8−) | ||||||||||||||||
| 190m3Tl | 410(lxx)# keV | >i μs | ix− | ||||||||||||||||
| 191Tl | 81 | 110 | 190.971786(8) | 20# min | β+ | 191Hg | (1/2+) | ||||||||||||
| 191mTl | 297(seven) keV | v.22(sixteen) min | β+ | 191Hg | 9/two(−) | ||||||||||||||
| 192Tl | 81 | 111 | 191.97223(3) | 9.6(4) min | β+ | 192Hg | (2−) | ||||||||||||
| 192m1Tl | 160(50) keV | 10.eight(2) min | β+ | 192Hg | (vii+) | ||||||||||||||
| 192m2Tl | 407(54) keV | 296(5) ns | (viii−) | ||||||||||||||||
| 193Tl | 81 | 112 | 192.97067(12) | 21.6(8) min | β+ | 193Hg | 1/2(+#) | ||||||||||||
| 193mTl | 369(four) keV | 2.11(15) min | IT (75%) | 193Tl | ix/2− | ||||||||||||||
| β+ (25%) | 193Hg | ||||||||||||||||||
| 194Tl | 81 | 113 | 193.97120(xv) | 33.0(five) min | β+ | 194Hg | ii− | ||||||||||||
| α (10−vii%) | 190Au | ||||||||||||||||||
| 194mTl | 300(200)# keV | 32.8(ii) min | β+ | 194Hg | (7+) | ||||||||||||||
| 195Tl | 81 | 114 | 194.969774(15) | 1.16(5) h | β+ | 195Hg | 1/ii+ | ||||||||||||
| 195mTl | 482.63(17) keV | 3.6(four) s | It | 195Tl | 9/2− | ||||||||||||||
| 196Tl | 81 | 115 | 195.970481(13) | 1.84(three) h | β+ | 196Hg | 2− | ||||||||||||
| 196mTl | 394.ii(5) keV | i.41(two) h | β+ (95.5%) | 196Hg | (7+) | ||||||||||||||
| IT (4.5%) | 196Tl | ||||||||||||||||||
| 197Tl | 81 | 116 | 196.969575(18) | 2.84(4) h | β+ | 197Hg | 1/2+ | ||||||||||||
| 197mTl | 608.22(8) keV | 540(ten) ms | IT | 197Tl | 9/two− | ||||||||||||||
| 198Tl | 81 | 117 | 197.97048(nine) | 5.3(five) h | β+ | 198Hg | 2− | ||||||||||||
| 198m1Tl | 543.5(4) keV | 1.87(3) h | β+ (54%) | 198Hg | 7+ | ||||||||||||||
| IT (46%) | 198Tl | ||||||||||||||||||
| 198m2Tl | 687.ii(5) keV | 150(forty) ns | (5+) | ||||||||||||||||
| 198m3Tl | 742.3(4) keV | 32.1(ten) ms | (10−)# | ||||||||||||||||
| 199Tl | 81 | 118 | 198.96988(three) | 7.42(8) h | β+ | 199Hg | ane/two+ | ||||||||||||
| 199mTl | 749.vii(iii) keV | 28.4(2) ms | IT | 199Tl | 9/2− | ||||||||||||||
| 200Tl | 81 | 119 | 199.970963(half dozen) | 26.1(i) h | β+ | 200Hg | two− | ||||||||||||
| 200m1Tl | 753.six(2) keV | 34.iii(10) ms | IT | 200Tl | 7+ | ||||||||||||||
| 200m2Tl | 762.0(ii) keV | 0.33(v) μs | 5+ | ||||||||||||||||
| 201Tl[n 8] | 81 | 120 | 200.970819(sixteen) | 72.912(17) h | EC | 201Hg | 1/ii+ | ||||||||||||
| 201mTl | 919.fifty(9) keV | ii.035(vii) ms | Information technology | 201Tl | (9/two−) | ||||||||||||||
| 202Tl | 81 | 121 | 201.972106(16) | 12.23(2) d | β+ | 202Hg | 2− | ||||||||||||
| 202mTl | 950.xix(10) keV | 572(7) μs | 7+ | ||||||||||||||||
| 203Tl | 81 | 122 | 202.9723442(xiv) | Observationally Stable [n nine] | 1/two+ | 0.2952(1) | 0.29494–0.29528 | ||||||||||||
| 203mTl | 3400(300) keV | 7.vii(v) μs | (25/ii+) | ||||||||||||||||
| 204Tl | 81 | 123 | 203.9738635(13) | 3.78(two) y | β− (97.1%) | 204Pb | 2− | ||||||||||||
| EC (ii.ix%) | 204Hg | ||||||||||||||||||
| 204m1Tl | 1104.0(4) keV | 63(2) μs | (7)+ | ||||||||||||||||
| 204m2Tl | 2500(500) keV | 2.vi(2) μs | (12−) | ||||||||||||||||
| 204m3Tl | 3500(500) keV | ane.half-dozen(ii) μs | (20+) | ||||||||||||||||
| 205Tl[n 10] | 81 | 124 | 204.9744275(14) | Observationally Stable [due north xi] | ane/2+ | 0.7048(1) | 0.70472–0.70506 | ||||||||||||
| 205m1Tl | 3290.63(17) keV | two.6(2) μs | 25/ii+ | ||||||||||||||||
| 205m2Tl | 4835.6(fifteen) keV | 235(10) ns | (35/2–) | ||||||||||||||||
| 206Tl | Radium Eastward | 81 | 125 | 205.9761103(15) | 4.200(17) min | β− | 206Pb | 0− | Trace[n 12] | ||||||||||
| 206mTl | 2643.11(xix) keV | 3.74(three) min | IT | 206Tl | (12–) | ||||||||||||||
| 207Tl | Actinium C | 81 | 126 | 206.977419(half-dozen) | iv.77(two) min | β− | 207Lead | i/2+ | Trace[northward 13] | ||||||||||
| 207mTl | 1348.1(iii) keV | 1.33(eleven) s | IT (99.9%) | 207Tl | 11/two– | ||||||||||||||
| β− (.1%) | 207Pb | ||||||||||||||||||
| 208Tl | Thorium C" | 81 | 127 | 207.9820187(21) | 3.053(4) min | β− | 208Pb | five+ | Trace[due north 14] | ||||||||||
| 209Tl | 81 | 128 | 208.985359(8) | ii.161(vii) min | β− | 209Atomic number 82 | i/two+ | Trace[n 15] | |||||||||||
| 210Tl | Radium C″ | 81 | 129 | 209.990074(12) | one.thirty(3) min | β− (99.991%) | 210Atomic number 82 | (5+)# | Trace[due north 12] | ||||||||||
| β−, northward (.009%) | 209Atomic number 82 | ||||||||||||||||||
| 211Tl | 81 | 130 | 210.993480(50) | lxxx(16) s | β− (97.8%) | 211Pb | 1/2+ | ||||||||||||
| β−, n (ii.2%) | 210Pb | ||||||||||||||||||
| 212Tl | 81 | 131 | 211.998340(220)# | 31(viii) s | β− (98.2%) | 212Atomic number 82 | (v+) | ||||||||||||
| β−, n (one.8%) | 211Pb | ||||||||||||||||||
| 213Tl | 81 | 132 | 213.001915(29) | 24(4) s | β− (92.4%) | 213Atomic number 82 | 1/2+ | ||||||||||||
| β−, northward (seven.6%) | 212Pb | ||||||||||||||||||
| 214Tl | 81 | 133 | 214.006940(210)# | xi(2) southward | β− (66%) | 214Lead | 5+# | ||||||||||||
| β−, n (34%) | 213Pb | ||||||||||||||||||
| 215Tl | 81 | 134 | 215.010640(320)# | x(4) s | β− (95.4%) | 215Pb | i/2+# | ||||||||||||
| β−, northward (4.half-dozen%) | 214Atomic number 82 | ||||||||||||||||||
| 216Tl | 81 | 135 | 216.015800(320)# | half-dozen(3) south | β− | 216Pb | 5+# | ||||||||||||
| β−, n (<xi.5%) | 215Pb | ||||||||||||||||||
| This table header & footer: | |||||||||||||||||||
- ^ thousandTl – Excited nuclear isomer.
- ^ ( ) – Uncertainty (1σ) is given in concise form in parentheses after the corresponding concluding digits.
- ^ # – Atomic mass marked #: value and uncertainty derived not from purely experimental data, but at least partly from trends from the Mass Surface (TMS).
- ^ a b c # – Values marked # are non purely derived from experimental data, but at least partly from trends of neighboring nuclides (TNN).
- ^ Modes of decay:
- ^ Assuming symbol as daughter – Daughter product is stable.
- ^ ( ) spin value – Indicates spin with weak assignment arguments.
- ^ Main isotope used in scintigraphy
- ^ Believed to undergo α disuse to 199Au
- ^ Concluding decay product of 4n+1 decay concatenation (the Neptunium series)
- ^ Believed to undergo α decay to 201Au
- ^ a b Intermediate decay product of 238U
- ^ Intermediate disuse product of 235U
- ^ Intermediate decay product of 232Th
- ^ Intermediate decay product of 237Np
Thallium-201 [edit]
Thallium-201 (201Tl) is a synthetic radioisotope of thallium. It has a half-life of 73 hours and decays by electron capture, emitting 10-rays (~70–80 keV), and photons of 135 and 167 keV in ten% full abundance.[10] Thallium-201 is synthesized by the neutron activation of stable thallium in a nuclear reactor,[10] [eleven] or by the 203Tl(p, 3n)201Atomic number 82 nuclear reaction in cyclotrons, as 201Atomic number 82 naturally decays to 201Tl afterwards.[12] It is a radiopharmaceutical, as information technology has skillful imaging characteristics without excessive patient radiation dose. It is the most popular isotope used for thallium nuclear cardiac stress tests.[13]
References [edit]
- ^ "Standard Diminutive Weights: Thallium". CIAAW. 2009.
- ^ Meija, Juris; et al. (2016). "Atomic weights of the elements 2013 (IUPAC Technical Report)". Pure and Practical Chemical science. 88 (iii): 265–91. doi:10.1515/pac-2015-0305.
- ^ "Thallium Enquiry". doe.gov. Department of Energy. Archived from the original on 2006-12-09. Retrieved 23 March 2018.
- ^ Manual for reactor produced radioisotopes from the International Diminutive Energy Agency
- ^ "Bound-state beta decay of highly ionized atoms" (PDF). Archived from the original (PDF) on Oct 29, 2013. Retrieved June nine, 2013.
- ^ Marcillac, P.; Coron, North.; Dambier, G.; et al. (2003). "Experimental detection of α-particles from the radioactive decay of natural bismuth". Nature. 422 (6934): 876–878. Bibcode:2003Natur.422..876D. doi:10.1038/nature01541. PMID 12712201. S2CID 4415582.
- ^ Half-life, decay way, nuclear spin, and isotopic composition is sourced in:
Audi, G.; Kondev, F. One thousand.; Wang, M.; Huang, West. J.; Naimi, S. (2017). "The NUBASE2016 evaluation of nuclear properties" (PDF). Chinese Physics C. 41 (3): 030001. Bibcode:2017ChPhC..41c0001A. doi:x.1088/1674-1137/41/three/030001. - ^ Wang, One thousand.; Audi, K.; Kondev, F. Thousand.; Huang, W. J.; Naimi, S.; Xu, X. (2017). "The AME2016 atomic mass evaluation (II). Tables, graphs, and references" (PDF). Chinese Physics C. 41 (3): 030003-1–030003-442. doi:10.1088/1674-1137/41/three/030003.
- ^ Reich, E. S. (2010). "Mercury serves up a nuclear surprise: a new type of fission". Scientific American . Retrieved 12 May 2011.
- ^ a b Audi, Georges; Bersillon, Olivier; Blachot, Jean; Wapstra, Aaldert Hendrik (2003), "The Due northUBASE evaluation of nuclear and decay properties", Nuclear Physics A, 729: three–128, Bibcode:2003NuPhA.729....3A, doi:10.1016/j.nuclphysa.2003.11.001
- ^ "Manual for reactor produced radioisotopes" (PDF). International Atomic Energy Agency. 2003. Archived (PDF) from the original on 2011-05-21. Retrieved 2010-05-13 .
- ^ Cyclotron Produced Radionuclides: Principles and Do (PDF). International Atomic Energy Agency. 2008. ISBN9789201002082 . Retrieved 2022-07-01 .
- ^ Maddahi, Jamshid; Berman, Daniel (2001). "Detection, Evaluation, and Hazard Stratification of Coronary Artery Illness by Thallium-201 Myocardial Perfusion Scintigraphy 155". Cardiac SPECT imaging (2nd ed.). Lippincott Williams & Wilkins. pp. 155–178. ISBN978-0-7817-2007-6. Archived from the original on 2017-02-22. Retrieved 2016-09-26 .
- Isotope masses from:
- Audi, Georges; Bersillon, Olivier; Blachot, Jean; Wapstra, Aaldert Hendrik (2003), "The NUBASE evaluation of nuclear and decay properties", Nuclear Physics A, 729: 3–128, Bibcode:2003NuPhA.729....3A, doi:10.1016/j.nuclphysa.2003.11.001
- Isotopic compositions and standard diminutive masses from:
- de Laeter, John Robert; Böhlke, John Karl; De Bièvre, Paul; Hidaka, Hiroshi; Peiser, H. Steffen; Rosman, Kevin J. R.; Taylor, Philip D. P. (2003). "Atomic weights of the elements. Review 2000 (IUPAC Technical Report)". Pure and Applied Chemistry. 75 (6): 683–800. doi:10.1351/pac200375060683.
- Wieser, Michael East. (2006). "Atomic weights of the elements 2005 (IUPAC Technical Study)". Pure and Practical Chemical science. 78 (xi): 2051–2066. doi:10.1351/pac200678112051.
- "News & Notices: Standard Atomic Weights Revised". International Union of Pure and Practical Chemistry. xix October 2005.
- Half-life, spin, and isomer data selected from the following sources.
- Audi, Georges; Bersillon, Olivier; Blachot, Jean; Wapstra, Aaldert Hendrik (2003), "The NUBASE evaluation of nuclear and decay backdrop", Nuclear Physics A, 729: 3–128, Bibcode:2003NuPhA.729....3A, doi:10.1016/j.nuclphysa.2003.11.001
- National Nuclear Information Heart. "NuDat 2.x database". Brookhaven National Laboratory.
- Holden, Norman Eastward. (2004). "11. Table of the Isotopes". In Lide, David R. (ed.). CRC Handbook of Chemistry and Physics (85th ed.). Boca Raton, Florida: CRC Press. ISBN978-0-8493-0485-9.
Thallium Has Two Stable Isotopes,
Source: https://en.wikipedia.org/wiki/Isotopes_of_thallium
Posted by: mcguiganselse2000.blogspot.com


0 Response to "Thallium Has Two Stable Isotopes"
Post a Comment